While the efficacy results recently reported by coronavirus vaccine makers are impressive, “the FDA doesn't authorize vaccines or approve any medical product, just on the basis of a press release,” Dr. Stephen Hahn, commissioner of the US Food and Drug Administration, said Tuesday.
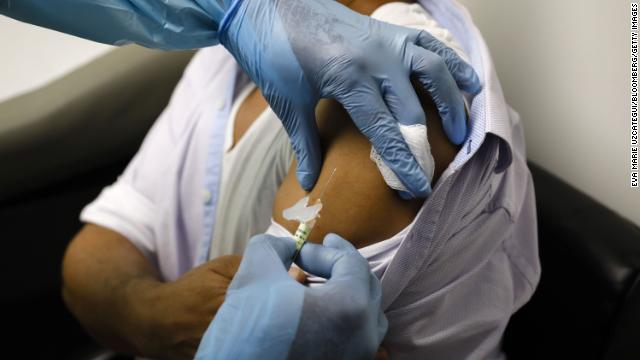
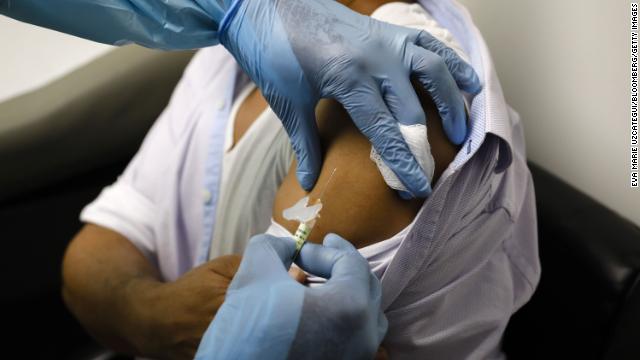 A health worker injects a person during clinical trials for a Covid-19 vaccine at Research Centers of America in Hollywood, Florida, on Sept. 9, 2020. Eva Marie Uzcategui/Bloomberg via Getty Images
A health worker injects a person during clinical trials for a Covid-19 vaccine at Research Centers of America in Hollywood, Florida, on Sept. 9, 2020. Eva Marie Uzcategui/Bloomberg via Getty Images
Pfizer submitted an application to the FDA last Friday requesting emergency use authorization of its coronavirus vaccine. So far, it’s released data only in a news release, but says the vaccine was 95% effective in preventing infection.
“Our scientists are going to pour over the data -- and remember, this is a study of over 44,000 individuals -- so we're going to look at all the patient data and be very careful about number crunching to make sure that we agree with the conclusion regarding safety and efficacy,” Hahn told South Carolina Sen. Tim Scott in an interview posted to Instagram.
The FDA’s Vaccines and Related Biological Products Advisory Committee will meet on Dec. 10 to discuss the data. Hahn said the public will be able to watch the meeting virtually, and a summary of the data will be available online.
“That committee is going to report back to us, and then after we hear their recommendations, we're going to move forward,” he said.
Hahn emphasized the FDA will not hesitate to make a decision “either up or down” based on the information available.
“We're going to use that process for every other application that comes forward, no matter what,” he added.










Share this page with your family and friends.